Generic name Nicorandil for Injection Ingredients & appearance Nicorandil, white loose mass or powder Indications: Unstable angina pectoris Acute heart failure (including acute exacerbation of chronic heart failure)
View detailsAmbroxol Hydrochloride Oral Solution
Generic name Ambroxol Hydrochloride Drops Ingredients & appearance In colorless or light-yellow clear solution with aroma. Indications: To thin down and break up phlegm (sputum) and is used to clear congestion in the treatment of respiratory diseases which have thick phlegm or too much phlegm
View detailsGeneric name Fudosteine Oral Solution Ingredients & appearance Fudosteine, in brown solution Indications: For expectoration during asthma, bronchitis, bronchiectasis, tuberculosis, pneumoconiosis, emphysema, atypical mycobacterial disease, diffuse panbronchiolitis.
View detailsSodium valproate oral solution
Generic name Sodium Valproate Oral Solution Ingredients & appearance Sodium valproate, in pink, transparent sticky liquid. Indications: For general, partial or other types of epilepsy.
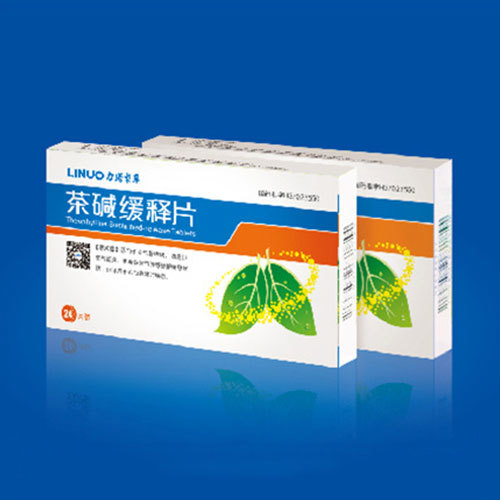
View detailsTheophylline Sustained Release Tablets
[Common Name] Theophylline Sustained Release Tablets [Ingredients] The main ingredient of this product is Theophylline. [Character] This product is a white tablet. [Indications] It is suitable for relieving wheezing symptoms such as bronchial asthma, wheezing bronchitis, and obstructive emphysema; it can also be used for wheezing in heart failure.
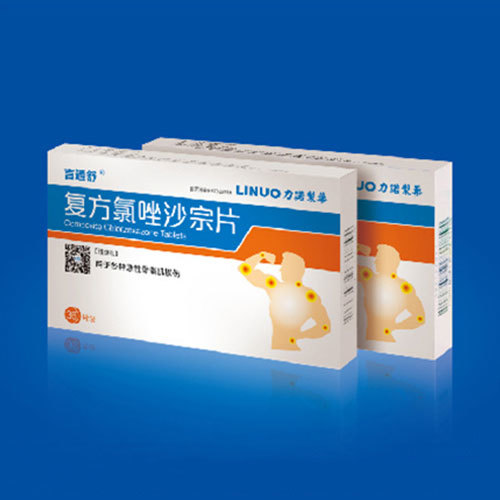
View details[Trade Name] Yantongshu [Common Name] Compound Chlorzoxazone Tablets [Ingredients] This product is a compound preparation, each tablet contains 0.125g of Chlorzoxazone and 0.15g of Acetaminophen. [Character] This product is a white tablet. [Indications] Used for various acute skeletal muscle injuries.
View detailsADD:#30766 Jingshidong Road, Linuo High-tech Park, Jinan, Shandong Province, P. R. China